An alien rodent on a protected island: estimating the abundance of the rock cavy on Fernando de Noronha, Brazil
DOI:
https://doi.org/10.37002/biodiversidadebrasileira.v15i1.2464Palavras-chave:
Kerodon rupestris , capture-resight , exotic species , island conservationResumo
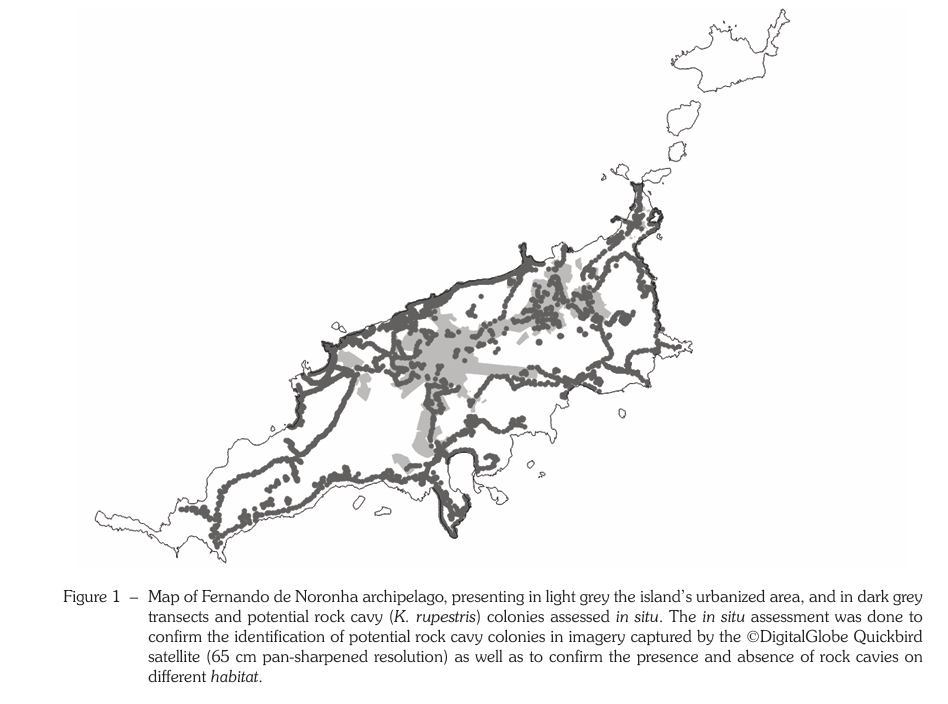
Understanding an alien species’ population structure and dynamics is crucial to both assess its potential threat level to native environments and to plan management if needed. This is achieved by estimating population parameters. However, on island environments – especially in developing countries – logistics, cost and certain species traits may hinder data collection, resulting in sparse datasets. The present study aimed at providing preliminary estimation of survival probability and abundance of rock cavy (Kerodon rupestris; Caviidae: Rodentia) on the island of Fernando de Noronha using a sparse data. Using a zero-truncated poisson log-normal mixed effects model (ZPNE) we first estimated the number of individuals in one rock cavy colony (i.e., Boldró colony). Using its calculated density (number of individuals divided by colony area) and information on mapped colonies collected using satellite imagery, we calculated the island population size. The ZPNE model presented a mean survival probability of 0.5499 in 185 days, and a mean capture probability of 0.858. The extrapolation of population size estimates from the Boldró colony (21; CI95% 12 – 36 individuals) suggests that the rock cavy population in Fernando de Noronha consists of 5,473 (CI95% 3,114 – 9,622) individuals. Even with limited data and warranted caution, the present preliminary study assessed population parameters for this insular rock cavy population, contributing with valuable information for planning its management for the first time.
Downloads
Referências
[1] Richardson DM, Pyšek P, Carlton JT. A Compendium of Essential Concepts and Terminology in Invasion Ecology. In: Richardson DM, editor. Fifty Years of Invasion Ecology: The Legacy of Charles Elton. 1st ed., Hoboken, NJ, USA: Blackwell Publishing Ltd; 2011, p. 409–20. DOI: https://doi.org/10.1002/9781444329988.ch30
[2] Davis MA, Chew MK, Hobbs RJ, Lugo AE, Ewel JJ, Vermeij GJ, et al. Don’t judge species on their origins. Nature 2011;474:153–4. doi: 10.1038/474153a. DOI: https://doi.org/10.1038/474153a
[3] Richardson DM, Pysek P, Rejmanek M, Barbour MG, Panetta FD, West CJ. Naturalization and invasion of alien plants: concepts and definitions. Diversity and Distributions 2000;6:93–107. doi: 10.1046/j.1472-4642.2000.00083.x. DOI: https://doi.org/10.1046/j.1472-4642.2000.00083.x
[4] Blackburn TM, Pyšek P, Bacher S, Carlton JT, Duncan RP, Jarošík V, et al. A proposed unified framework for biological invasions. Trends in Ecology & Evolution 2011;26:333–9. doi: 10.1016/j.tree.2011.03.023. DOI: https://doi.org/10.1016/j.tree.2011.03.023
[5] Simberloff D, Martin JJ-L, Genovesi P, Maris V, Wardle D a, Aronson J, et al. Impacts of biological invasions: what’s what and the way forward. Trends in Ecology & Evolution 2013;28:58–66. doi: 10.1016/j.tree.2012.07.013. DOI: https://doi.org/10.1016/j.tree.2012.07.013
[6] Parker IM, Simberloff D, Lonsdale WM, Goodell K, Wonham M, Kareiva PM, et al. Impact: Toward a Framework for Understanding the Ecological Effects of Invaders. Biological Invasions 1999;1:3–19. doi: 10.1023/A:1010034312781. DOI: https://doi.org/10.1023/A:1010034312781
[7] Stephenson PJ, Ntiamoa-Baidu Y, Simaika JP. The Use of Traditional and Modern Tools for Monitoring Wetlands Biodiversity in Africa: Challenges and Opportunities. Front Environ Sci 2020;8:61. doi: 10.3389/fenvs.2020.00061. DOI: https://doi.org/10.3389/fenvs.2020.00061
[8] Baker CM, Bode M. Recent advances of quantitative modeling to support invasive species eradication on islands. Conservat Sci and Prac 2021;3. doi: 10.1111/csp2.246. DOI: https://doi.org/10.1111/csp2.246
[9] Schulz-Neto A. Observando aves no Parque Nacional Marinho de Fernando de Noronha: guía de campo. Brasília, DF: Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis; 1995.
[10] Oren D. Resultados de uma nova expedição zoológica a Fernando de Noronha. Boletim Do Museu Paraense Emílio Goeldi 1984;Zoologia:19–44.
[11] Alves ML, Leite LMRM. Distribuição geográfica do Kerodon rupestris (Mammalia, Rodentia) em Fernando de Noronha, PE, Brasil. In: Overal W, editor. Resumos XII Congresso Latino-Americano de Zoologia / XIX Congresso Brasileiro de Zoologia., Belem: Sociedade Brasileira de Zoologia; 1992, p. 156.
[12] Conceição AM, Bocchiglieri A. Population density and use of space by Kerodon rupestris: An endemic and threatened rodent in the semiarid areas of Brazil. Journal of Arid Environments 2021;186:104425. doi: 10.1016/j.jaridenv.2020.104425. DOI: https://doi.org/10.1016/j.jaridenv.2020.104425
[13] Lacher T. Rates of growth in Kerodon rupestris and an assessment of its potential as a domesticated food source. Papéis Avulsos de Zoologia (São Paulo) 1979;33:67–76. DOI: https://doi.org/10.11606/0031-1049.1979.33.p67-76
[14] Zappes I, Portella A, Lessa G. Description of Karyotype of Kerodon acrobata, an endemic rodent in Brazilian Cerrado. Braz J Biol 2014;74:251–6. doi: 10.1590/1519-6984.23512. DOI: https://doi.org/10.1590/1519-6984.23512
[15] Lessa G, Gonçalves PR, Pessôa LM. Variação geográfica em caracteres cranianos quantitativos de Kerodon rupestris (WIED, 1820) (RODENTIA, CAVIIDAE). Arquivos do Museu Nacional 2005;63:75–88.
[16] Freitas RR, Rocha PLB da, Simões-Lopes PC. Habitat structure and small mammals abundances in one semiarid landscape in the Brazilian Caatinga. Rev Bras Zool 2005;22:119–29. doi: 10.1590/S0101-81752005000100015. DOI: https://doi.org/10.1590/S0101-81752005000100015
[17] Sousa RA, Menezes AAL. Circadian rhythm of motor activity of the Brazilian rock cavy ( Kerodon rupestris ) under artificial photoperiod. Biological Rhythm Research 2006;37:443–50. doi: 10.1080/09291010600869836. DOI: https://doi.org/10.1080/09291010600869836
[18] Xavier SCC, Vaz VC, D’Andrea PS, Herrera L, Emperaire L, Alves JR, et al. Mapping of the distribution of Trypanosoma cruzi infection among small wild mammals in a conservation unit and its surroundings (Northeast-Brazil). Parasitology International 2007;56:119–28. doi: 10.1016/j.parint.2007.01.003. DOI: https://doi.org/10.1016/j.parint.2007.01.003
[19] Delciellos AC. Mammals of four Caatinga areas in northeastern Brazil: inventory, species biology, and community structure. Cl 2016;12:1916. doi: 10.15560/12.3.1916. DOI: https://doi.org/10.15560/12.3.1916
[20] UNESCO. Brazilian Atlantic Islands: Fernando de Noronha and Atol das Rocas Reserves 2001.
[21] Serafini TZ, França GB de, Andriguetto-Filho JM. Ilhas oceânicas brasileiras: biodiversidade conhecida e sua relação com o histórico de uso e ocupação humana. RGCI 2010;10:281–301. doi: 10.5894/rgci178. DOI: https://doi.org/10.5894/rgci178
[22] Mello TJ, Oliveira AAD. Making a Bad Situation Worse: An Invasive Species Altering the Balance of Interactions between Local Species. PLoS ONE 2016;11:e0152070. doi: 10.1371/journal.pone.0152070. DOI: https://doi.org/10.1371/journal.pone.0152070
[23] Barcelos D, Vieira EM, Pinheiro MS, Ferreira GB. A before−after assessment of the response of mammals to tourism in a Brazilian national park. Oryx 2022;56:854–63. doi: 10.1017/S0030605321001472. DOI: https://doi.org/10.1017/S0030605321001472
[24] Mares M, Lacher TE. Ecological, morphological and behavioral convergence in rock-dwelling mammals. Current Mammalogy 1987;1:307–48. DOI: https://doi.org/10.1007/978-1-4757-9909-5_8
[25] Oliveira FG, Nascimento-Júnior ESD, Cavalcante JC, Guzen FP, Cavalcante JDS, Soares JG, et al. Topographic specializations of catecholaminergic cells and ganglion cells and distribution of calcium binding proteins in the crepuscular rock cavy ( Kerodon rupestris ) retina. Journal of Chemical Neuroanatomy 2018;90:57–69. doi: 10.1016/j.jchemneu.2017.12.007. DOI: https://doi.org/10.1016/j.jchemneu.2017.12.007
[26] Lacher T. The comparative social behavior of Kerodon rupestris and Galea spixii and the evolution of behavior in the Caviidae. vol. 17. Pittsburgh: Carnegie Museum of Natural History; 1981. DOI: https://doi.org/10.5962/p.228596
[27] Nutt K. Socioecology of rock-dwelling rodents. In: Wolff Jerry, Sherman PW, editors. Rodent societies: an ecological and evolutionary perspective, Chicago and London: University of Chicago Press; 2007, p. 416–26.
[28] IUCN. The IUCN Red List of Threatened Species 2016.
[29] Oliveira J, Bonvicino C. Ordem Rodentia. Mamíferos do Brasil, Londrina, Brazil: Universidade Estadual de Londrina; 2006, p. 347–406.
[30] Lacher TE, Willig MR, Mares MA. Food Preference as a Function of Resource Abundance with Multiple Prey Types: An Experimental Analysis of Optimal Foraging Theory. The American Naturalist 1982;120:297–316. DOI: https://doi.org/10.1086/283992
[31] Roberts M, Maliniak E, Deal M. The reproductive biology of the rock cavy, Kerodon rupestris, in captivity: A study of reproductive adaptation in a trophic specialist. Mammalia 1984;48. doi: 10.1515/mamm.1984.48.2.253. DOI: https://doi.org/10.1515/mamm.1984.48.2.253
[32] Tasse J. Maternal and paternal care in the rock cavy,Kerodon rupestris, a South American hystricomorph rodent. Zoo Biol 1986;5:27–43. doi: 10.1002/zoo.1430050105. DOI: https://doi.org/10.1002/zoo.1430050105
[33] Willig MR, Lacher TE. Food selection of a tropical mammalian folivore in relation to leaf-nutrient content. Journal of Mammalogy 1991;72:314–21. DOI: https://doi.org/10.2307/1382101
[34] De Matos Dias D, De Campos CB, Guimarães Rodrigues FH. Behavioural ecology in a predator-prey system. Mammalian Biology 2018;92:30–6. doi: 10.1016/j.mambio.2018.04.005. DOI: https://doi.org/10.1016/j.mambio.2018.04.005
[35] Conceição AM, Bocchiglieri A. Temporal variation in the diet of the endemic and threatened rodent Kerodon rupestris in the semiarid area of Brazil. Mammalia 2021;85:537–40. doi: 10.1515/mammalia-2021-0043. DOI: https://doi.org/10.1515/mammalia-2021-0043
[36] da Silva Neto EJ. Morphology of the regiones ethmoidalis and orbitotemporalis in Galea musteloides Meyen 1832 and Kerodon rupestris (Wied-Neuwied 1820) (Rodentia: Caviidae) with comments on the phylogenetic systematics of the Caviidae. Journal of Zoological Systematics and Evolutionary Research 2000;38:219–29. doi: 10.1046/j.1439-0469.2000.384135.x. DOI: https://doi.org/10.1046/j.1439-0469.2000.384135.x
[37] Oliveira M, Carter A, Bonatelli M, Ambrosio CE, Miglino MA. Placentation in the rock cavy, Kerodon rupestris (Wied). Placenta 2006;27:87–97. doi: 10.1016/j.placenta.2004.11.012. DOI: https://doi.org/10.1016/j.placenta.2004.11.012
[38] Nascimento ES, Souza APM, Duarte RB, Magalhães MAF, Silva SF, Cavalcante JC, et al. The suprachiasmatic nucleus and the intergeniculate leaflet in the rock cavy (Kerodon rupestris): Retinal projections and immunohistochemical characterization. Brain Research 2010;1320:34–46. doi: 10.1016/j.brainres.2010.01.034. DOI: https://doi.org/10.1016/j.brainres.2010.01.034
[39] Soares JG, Cavalcanti JRLP, Oliveira FG, Pontes ALB, Sousa TB, Freitas LM, et al. Nuclear organization of the serotonergic system in the brain of the rock cavy (Kerodon rupestris). Journal of Chemical Neuroanatomy 2012;43:112–9. doi: 10.1016/j.jchemneu.2012.03.001. DOI: https://doi.org/10.1016/j.jchemneu.2012.03.001
[40] Cavalcanti JRLP, Soares JG, Oliveira FG, Guzen FP, Pontes ALB, Sousa TB, et al. A cytoarchitectonic and TH-immunohistochemistry characterization of the dopamine cell groups in the substantia nigra, ventral tegmental area and retrorubral field in the rock cavy (Kerodon rupestris). Journal of Chemical Neuroanatomy 2014;55:58–66. doi: 10.1016/j.jchemneu.2014.01.002. DOI: https://doi.org/10.1016/j.jchemneu.2014.01.002
[41] de Medeiros Silva A, de Santana MAD, de Góis Morais PLA, de Sousa TB, Januário Engelberth RCG, de Souza Lucena EE, et al. Serotonergic fibers distribution in the midline and intralaminar thalamic nuclei in the rock cavy (Kerodon rupestris). Brain Research 2014;1586:99–108. doi: 10.1016/j.brainres.2014.08.047. DOI: https://doi.org/10.1016/j.brainres.2014.08.047
[42] Santos AC dos, Aro MM de, Bertassoli BM, Viana DC, Vasconcelos BG, Rici REG, et al. Morphological characteristics of the tongue of the rock cavy- Kerodon rupestris wied, 1820 (Rodentia, caviidae). Bioscience Journal 2015;31:1174–82. doi: 10.14393/BJ-v31n4a2015-26061. DOI: https://doi.org/10.14393/BJ-v31n4a2015-26061
[43] Santos F, Magalhaes‐Junior JT, De Oliveira Carneiro I, Lambert SM, Da Silva Souza BMP, De Pauda AD, et al. Wild mammals involved in the transmission of Trypanosoma cruzi and food sources of Triatoma sherlocki in an endemic region of northeastern Brazil. Medical Vet Entomology 2023;37:396–406. doi: 10.1111/mve.12641. DOI: https://doi.org/10.1111/mve.12641
[44] Almeida CE, Faucher L, Lavina M, Costa J, Harry M. Molecular Individual-Based Approach on Triatoma brasiliensis: Inferences on Triatomine Foci, Trypanosoma cruzi Natural Infection Prevalence, Parasite Diversity and Feeding Sources. PLoS Negl Trop Dis 2016;10:e0004447. doi: 10.1371/journal.pntd.0004447. DOI: https://doi.org/10.1371/journal.pntd.0004447
[45] Labruna MB, Nava S, Marcili A, Barbieri ARM, Nunes PH, Horta MC, et al. A new argasid tick species (Acari: Argasidae) associated with the rock cavy, Kerodon rupestris Wied-Neuwied (Rodentia: Caviidae), in a semiarid region of Brazil. Parasites Vectors 2016;9:511. doi: 10.1186/s13071-016-1796-7. DOI: https://doi.org/10.1186/s13071-016-1796-7
[46] Resende NR, Soares Filho PL, Peixoto PPA, Silva AM, Silva SF, Soares JG, et al. Nuclear organization and morphology of cholinergic neurons in the brain of the rock cavy (Kerodon rupestris) (Wied, 1820). Journal of Chemical Neuroanatomy 2018;94:63–74. doi: 10.1016/j.jchemneu.2018.09.001. DOI: https://doi.org/10.1016/j.jchemneu.2018.09.001
[47] Fundação Oswaldo Cruz, Souza MVD, Chaves SADM, Hugot J-P, Iñiguez AM. NEW PARASITE RECORDS FROM Kerodon rupestris (RODENTIA, CAVIIDAE) AN ENDEMIC SPECIES TO NORTHEASTERN BRAZIL. Oecol Ast 2020;24:196–203. doi: 10.4257/oeco.2020.2401.18. DOI: https://doi.org/10.4257/oeco.2020.2401.18
[48] Lima-Neiva V, Toma HK, Abrantes Aguiar LM, Lopes CM, Dias LP, Monte Gonçalves TC, et al. The connection between Trypanosoma cruzi transmission cycles by Triatoma brasiliensis brasiliensis: A threat to human health in an area susceptible to desertification in the Seridó, Rio Grande do Norte, Brazil. PLoS Negl Trop Dis 2021;15:e0009919. doi: 10.1371/journal.pntd.0009919. DOI: https://doi.org/10.1371/journal.pntd.0009919
[49] Souza MV, Chaves SAM, Iñiguez AM. New observations from the intestinal fauna of Kerodon rupestris (Wied, 1820) (Rodentia, Cavidae), Brazil: a checklist spanning 30,000 years of parasitism. Braz J Biol 2021;81:989–98. doi: 10.1590/1519-6984.232838. DOI: https://doi.org/10.1590/1519-6984.232838
[50] Saldanha BM, Chame M, Nunes GKM, Sianto L, Leles D. Parasites of the Brazilian Rock Cavy, Kerodon rupestris: Revealing Their History in the Brazilian Semiarid Region. Journal of Parasitology 2022;108:395–402. doi: 10.1645/20-56. DOI: https://doi.org/10.1645/20-56
[51] Hutto RL, Pletschet SM, Hendricks P. A Fixed-radius Point Count Method for Nonbreeding and Breeding Season Use. The Auk 1986;103:593–602. doi: 10.1093/auk/103.3.593. DOI: https://doi.org/10.1093/auk/103.3.593
[52] McClintock BT, White GC, Antolin MF, Tripp DW. Estimating abundance using mark-resight when sampling is with replacement or the number of marked individuals is unknown. Biometrics 2009;65:237–46. doi: 10.1111/j.1541-0420.2008.01047.x. DOI: https://doi.org/10.1111/j.1541-0420.2008.01047.x
[53] McClintock BT, White GC. From NOREMARK to MARK: software for estimating demographic parameters using mark–resight methodology. Journal of Ornithology 2012;152:641–50. doi: 10.1007/s10336-010-0524-x. DOI: https://doi.org/10.1007/s10336-010-0524-x
[54] Adrian O, Sachser N. Diversity of social and mating systems in cavies: a review. Journal of Mammalogy 2011;92:39–53. doi: 10.1644/09-MAMM-S-405.1. DOI: https://doi.org/10.1644/09-MAMM-S-405.1
[55] Gaiotto JV, Abrahão CR, Dias RA, Bugoni L. Diet of invasive cats, rats and tegu lizards reveals impact over threatened species in a tropical island. Perspectives in Ecology and Conservation 2020;18:294–303. doi: 10.1016/j.pecon.2020.09.005. DOI: https://doi.org/10.1016/j.pecon.2020.09.005
[56] Quantum GIS Development Team. Development Team, 2012. Quantum GIS Geographic Information System. Open Source Geospatial Foundation Project. Free Software Foundation, India 2015.
[57] Poland TM, Juzwik J, Rowley A, Huebner CD, Kilgo JC, Lopez VM, et al. Management of Landscapes for Established Invasive Species. In: Poland TM, Patel-Weynand T, Finch DM, Miniat CF, Hayes DC, Lopez VM, editors. Invasive Species in Forests and Rangelands of the United States, Cham: Springer International Publishing; 2021, p. 133–84. doi: 10.1007/978-3-030-45367-1_7. DOI: https://doi.org/10.1007/978-3-030-45367-1_17
[58] da Rosa CA, de Almeida Curi NH, Puertas F, Passamani M. Alien terrestrial mammals in Brazil: current status and management. Biological Invasions 2017. doi: 10.1007/s10530-017-1423-3. DOI: https://doi.org/10.1007/s10530-017-1423-3
[59] Lilioso M, Reigada C, Pires-Silva D, Fontes FVHM, Limeira C, Monsalve-Lara J, et al. Dynamics of food sources, ecotypic distribution and Trypanosoma cruzi infection in Triatoma brasiliensis from the northeast of Brazil. PLoS Negl Trop Dis 2020;14:e0008735. doi: 10.1371/journal.pntd.0008735. DOI: https://doi.org/10.1371/journal.pntd.0008735
[60] Miller Jr, Turner M, Smithwick EAH, Dent CL, Stanley EH. Spatial Extrapolation: The Science of Predicting Ecological Patterns and Processes. BioScience. 2004 Apr 1;54(4):310–20. https://doi.org/10.1641/0006-3568(2004)054[0310:SETSOP]2.0.CO;2 DOI: https://doi.org/10.1641/0006-3568(2004)054[0310:SETSOP]2.0.CO;2
[61] Seymour A, Varnham K, Roy S, Harris S, Bhageerutty L, Church S, et al. Mechanisms underlying the failure of an attempt to eradicate the invasive Asian musk shrew Suncus murinus from an island nature reserve. Biological Conservation. 2005 Sep;125(1):23–35. https://doi.org/10.1016/j.biocon.2005.03.005 DOI: https://doi.org/10.1016/j.biocon.2005.03.005
[62] Russell JC, Holmes ND. Tropical island conservation: Rat eradication for species recovery. Biological Conservation. 2015 May;185:1–7. https://doi.org/10.1016/j.biocon.2015.01.009 DOI: https://doi.org/10.1016/j.biocon.2015.01.009
[63] Breslow N. Tests of Hypotheses in Overdispersed Poisson Regression and other Quasi-Likelihood Models. Journal of the American Statistical Association. 1990 Jun;85(410):565–71. https://doi.org/10.1080/01621459.1990.10476236 DOI: https://doi.org/10.1080/01621459.1990.10476236
[64] Schmutz JA, Ward DH, Sedinger JS, Rexstad EA. Survival estimation and the effects of dependency among animals. Journal of Applied Statistics. 1995 Nov;22(5–6):673–82. https://doi.org/10.1080/02664769524531 DOI: https://doi.org/10.1080/02664769524531
Downloads
Publicado
Edição
Seção
Licença
Copyright (c) 2025 Os autores mantêm os direitos autorais de seus artigos sem restrições, concedendo ao editor direitos de publicação não exclusivos.

Este trabalho está licenciado sob uma licença Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Os artigos estão licenciados sob uma licença Creative Commons Atribuição-NãoComercial-SemDerivações 4.0 Internacional (CC BY-NC-ND 4.0). O acesso é livre e gratuito para download e leitura, ou seja, é permitido copiar e redistribuir o material em qualquer mídia ou formato.











